THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
Reduce turnaround time and simplify laboratory workflow with a rapid full-spectrum sequencing single-assay thalassemia DNA screening solution that eliminates multiple parallel test protocols.
Challenges in thalassemia diagnosis
Thalassemia is typically caused by sequence variants in the HBA1, HBA2 or HBB genes. The common sequence variants include Single Nucleotide Variants (SNVs), smaller insertion and deletions (indels) as well as large, exon spanning Copy Number Variations (CNVs). A range of different techniques such as GAP-PCR, Sanger sequencing, reverse hybridisation and MLPA is traditionally required to assess all variants. Testing for both alpha thalassemia and beta thalassemia can be a complex process.
Workflows are laboratory-specific and often require the use of several different techniques to obtain a result. Relying on a patchwork of methods presents challenges such as:
- Long turn-around times to get an overview of both alpha and beta thalassemia sequence variants in a patient
- Resource-intensive and costly processes to validate, maintain and train operators on all assay types
- Risk that sequence variants remain undetected if the workflow is terminated when a first sequence variant is found, or a method is used where only sequence variant-specific detection is possible
- Risk for sample contamination and mix-up when handling multiple tubes and protocols
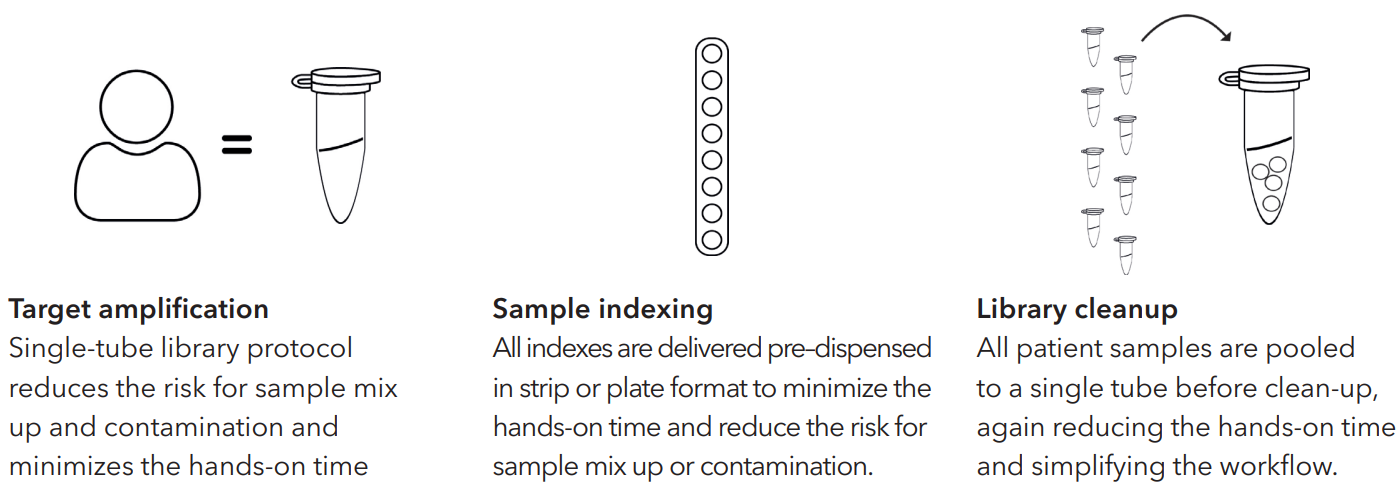
One assay for all thalassemia needs
Devyser thalassemia NGS* is a one-size-fits-all genetic sequencing test solution that robustly detects variants related to thalassemia in a rapid process. The kit identifies, single nucleotide variants, copy number variants and allows for direct detection of 17 major alpha and beta thalassemia deletions in a fast workflow that takes under five hours from DNA to start sequencing.
- Less than 45 minutes HOT
- Single tube NGS assay
- Sequences HBA1, HBA2 and HBB genes
The unique end-to-end procedure eliminates the traditional need for multiple parallel testing protocols, meeting growing market demand for rapid, convenient screening.
Fast and simple NGS workflow is suitable for any lab, whether running advanced genetic testing or large-scale mutation screening.
Improve your thalassemia testing workflow with:
- Simple & fast NGS testing
- Streamlined thalassemia workflow
- Analysis software available
Learn more about Devyser thalassemia NGS
View the thalassemia NGS Flyer
View the thalassemia NGS Brochure
View the Devyser addressing hemoglobinopathy using NGS webinar
*Research Use Only, please check regulatory status in your country.