Products are for professional/laboratory use only. ImmunoCAP component from Thermo Fisher – Allergen Component rSes i 1 Sesame seed.
Sesame allergy – a high risk condition
Sesame allergic patients have a high risk of experiencing severe allergic reactions. It has been reported to be even higher than for peanut and tree nut for some allergic patients.1,2 Sesame allergy is often severe, life-long and co-exists with tree nut allergy.2
Ses i 1 is a major sesame allergen in the 2S albumin seed storage protein group. These proteins are typically heat and digestion stable, and consequently a risk factor for severe reactions in sensitized individuals. Sesame allergy is often severe, life-long and co-exist with tree nut allergy. Among common seeds and nuts, it has been reported to cause allergic symptoms with the highest severity. Sesame is used in foods as seeds, oil, paste and flour, and is often a hidden allergen, leading to a high risk of accidental intake that may result in severe reactions.
Discover ImmunoCAP Allergen f449 - Allergen Component rSes i 1 Sesame seed test
View Phadia's range of instruments
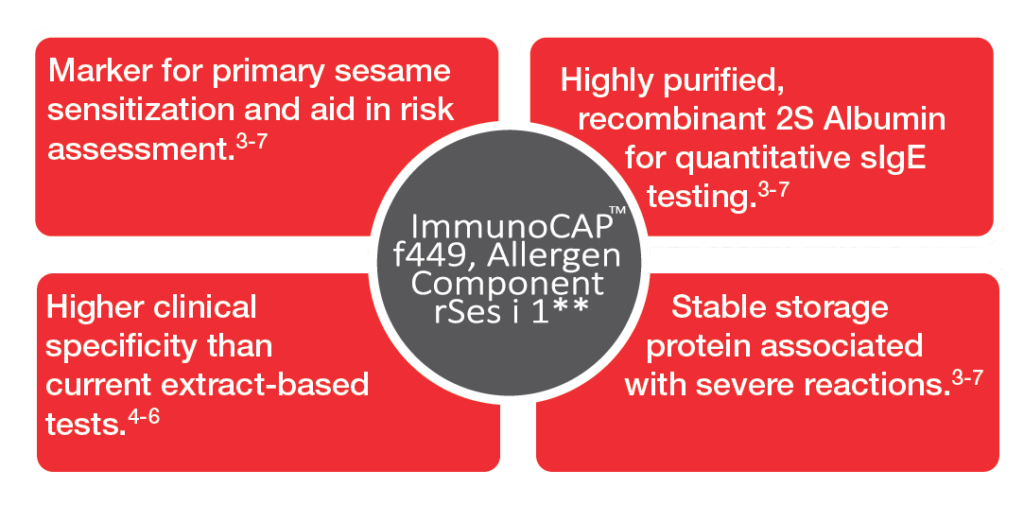
The new ImmunoCAP Allergen Component test provides:
- Marker for primary sesame allergy with higher clinical specificity than current extract-based tests
- Aid in the diagnosis and management of patients with primary sesame allergy.3-7
- Help assessing risk for severe events by providing quantitative sIgE levels to Ses i 1.3-7
- Higher clinical specificity than skin prick test and whole allergen sIgE test with component-resolved diagnostics.4-7
- First quantitative, CE marked IVD test on the market.
|
Product description |
Article number |
Barcode |
|---|---|---|
|
ImmunoCAP™ Allergen f449, Allergen Component rSes i 1 Sesame seed |
PU14610901 |
E7M |
View the allergen encyclopaedia
View Phadia's range of instruments
References
- Adatia A, Clarke AE, Yanishevsky Y, Ben-Shoshan M. Sesame allergy: current perspectives.
J Asthma Allergy. 2017;10:141-51. - Brough HA, Caubet JC, Mazon A, Haddad D, Bergmann MM, Wassenberg J et al. Defining challenge-proven coexistent nut and sesame seed allergy: A prospective multicenter European study. J Allergy Clin Immunol. 2020;145(4):1231-39.
- Maruyama N et al. Clin Exp Allergy. 2016;46(1):163-71.
- Yanagida N et al. J Allergy Clin Immunol Pract. 2019;7(6):2084-86.
- Saf S et al. J Allergy Clin Immunol Pract. 2020;8(5):1681-88.
- Goldberg MR et al. Pediatr Allergy Immunol. 2021 May
- doi: 10.1111/pai.13533. Online ahead of print. 5. Nachshon L et al. J Allergy Clin Immunol Pract. 2019;7:2775-81.