Biocartis Idylla™ GeneFusion Panel
Products are for professional/laboratory use only
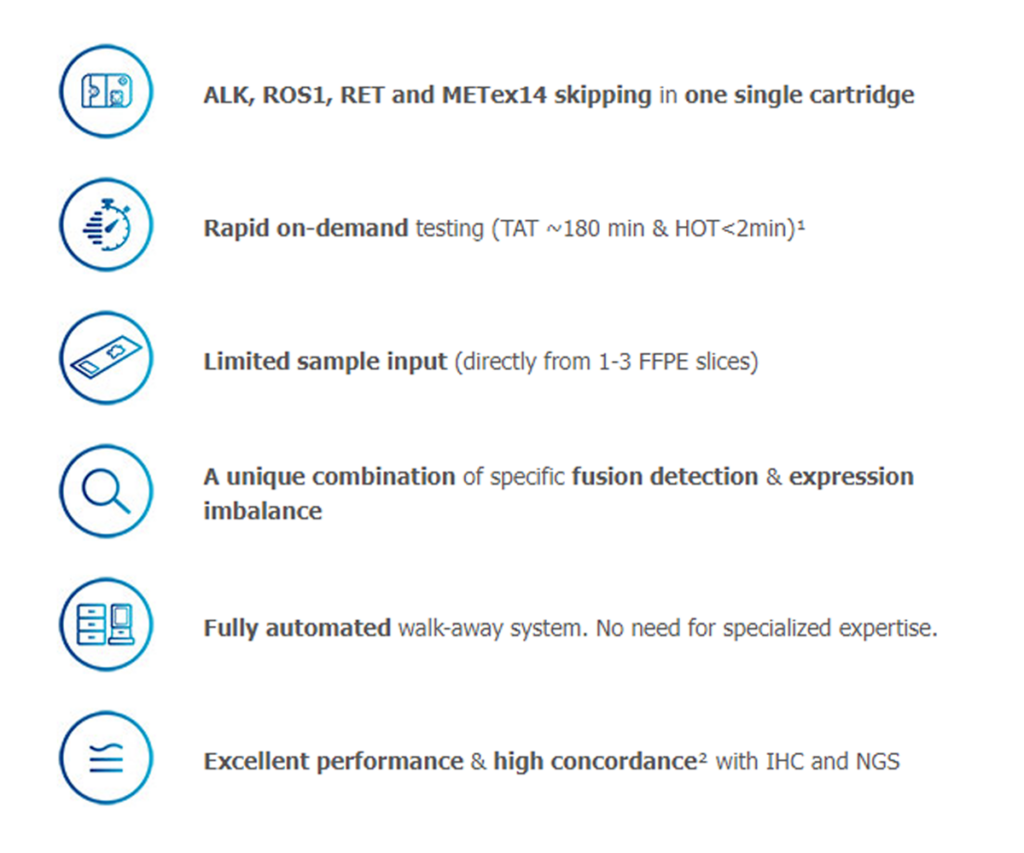
Within 180 minutes and with less than 2 minutes hands-on time, the fully automated Idylla™ GeneFusion cartridge detects ALK, ROS1 and RET fusions & MET exon 14 skipping in a single cartridge using only 1-3* FFPE tissue sections from patients with NSCLC.
*Check specimen requirements here
The Panel detects in one single cartridge ALK, ROS1, RET and METex14 skipping, a wide range of actionable targets relevant in non-small cell lung cancer (NSCLC). Designed for use in clinical laboratories, the Panel provides comprehensive testing results within 180 minutes, significantly faster than currently available testing methods which often take days or even weeks before results are available.
10% to 20% of advanced lung cancer patients don’t receive the appropriate targeted therapy due to slow turnaround time of many current testing methods, leading to a delayed time-to-treatment3
Lung cancer remains the leading cause of cancer deaths, with NSCLC being the most common type of lung cancer. The survival rate has increased over the past years due to the rapidly evolving treatment landscape for NSCLC. Gene fusions represent an important class of gene rearrangements and have become important in NSCLC, as they are linked to responses to certain targeted therapies. Their accurate and fast detection is critical to guide therapy choices, which is the reason why testing for gene rearrangements such as gene fusions is included in international NSCLC testing guidelines (including ESMO and NCCN).
However, comprehensive testing of actionable gene rearrangements in NSCLC is often complex and can require different technologies4. In order to test all needed biomarkers, laboratories usually have to use different instruments which are often not available within their own lab. Using different instruments also requires having enough biopsy samples of sufficient good quality, which can be difficult to obtain, especially in NSCLC patients.
The Idylla™ GeneFusion Panel consolidates traditional testing workflows into one streamlined, fully-automated process which provides reliable information on ALK, ROS1, RET and METex14 skipping and delivers results within 180 minutes. Moreover, the Panel only requires a limited amount of sample, thereby saving valuable tissue specimens. The Idylla™ GeneFusion Panel demonstrated high concordance results in a clinical comparison study where ALK was compared with IHC, and ROS1, RET and METex14 skipping were compared with NGS2.
DETECTION OF KNOWN AND NOVEL FUSIONS
The new Idylla™ GeneFusion Panel is a unique combination of two detection technologies:
- Gene fusion specific and METex14 skipping detection: Highly sensitive detection of known skipping isoforms by RT-qPCR of sample RNA.
- Expression Imbalance Analysis: Detection of fusion events without knowledge of the fusion partners by analysing expression ratios between the 5’ and 3’ ends of the gene. Expression imbalance results are indicative for the presence of a fusion or other rearrangements that lead to kinase domain expression and should be confirmed with another technology.
SPECIMEN REQUIREMENTS
One of the biggest challenges in oncology biomarker testing is the ability to obtain samples of sufficient size and quality. With the Idylla™ system only a minimal amount of sample is needed:
- If ≥ 20 mm² tissue area -> 1 x 5 μm FFPE tissue section (in 90% of all cases)
- If < 20 mm² tissue area -> 3 x 5 μm FFPE tissue sections
- ≥ 10% neoplastic cell content
Check regulatory status in your country.
References
¹ TAT: turnaround time – HOT: hands-on time
² The clinical performance evaluation compared the Idylla™ GeneFusion Panel with IHC (VENTANA ALK (D5F3) Assay, Roche Diagnostics GmbH) for ALK. ROS1, RET and METex14 skipping were evaluated versus NGS (Oncomine™ Focus Assay, Thermo Fisher Scientific).
3 Finall et al. Integration of rapid PCR testing as an adjunct to NGS in diagnostic pathology services within the UK: evidence from a case series of non-squamous, non-small cell lung cancer (NSCLC) patients with follow-up? J Clin Pathol. 5 Jan 2022 Chu et al. Clinical Utility and Performance of an Ultrarapid Multiplex RNA-Based Assay for Detection of ALK, ROS1, RET, and NTRK1/2/3 Rearrangements and MET Exon 14 Skipping Alterations. J Mol Diagn. 2022 Apr
4 i.e. gene fusions and METex14 skipping. Techniques used to detect NTRK gene fusions include DNA-based next-generation sequencing (NGS), RNA-based NGS, reverse-transcriptase PCR (RT-PCR), fluorescence in situ hybridisation (FISH), and immunohistochemistry (IHC). Source: OncologyPro, ESMO, see here, last consulted on 2 June 2022