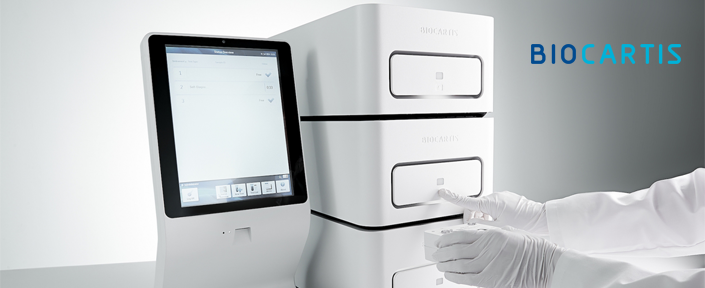
Biocartis – Clinical utility of Idylla EGFR
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
Within 150 minutes and with less than 2 minutes hands-on time, the fully automated Idylla™ EGFR Mutation Test covers 51 mutations in exons 18–21 in a single cartridge