Idylla™ EGFR Study Shows Reduction of Time-to-Treatment by 48% for Lung Cancer Patients
THESE PRODUCTS ARE NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC.
A recent publication of a large prospective study1 demonstrates the Idylla™ EGFR Mutation Test leads to:
- Significant reduction in time-to-treatment by 48% or on average 16.8 days faster than NGS
- Improved strategic treatment decisions within a multidisciplinary team
The study was performed on 238 samples which were tested both using a next generation sequencing (NGS) panel and the Idylla™ EGFR Mutation Test. The study showed:
- Concordance of 98.7% between the Idylla™ EGFR Mutation Test and the NGS panel
- Turnaround time was faster for the Idylla™ EGFR Mutation Test by an average of 12.4 days
- In the EGFR positive cohort, the Idylla™ EGFR Mutation Test led to a 48% reduction and on average 16.8 days faster time-to-treatment
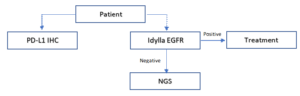
Furthermore, the study concludes that the Idylla™ EGFR Mutation Test could contribute to overall time and cost savings for patients if testing is implemented in a stepwise manner:
Such first-line use of Idylla™ EGFR Mutation Test allows to obtain EGFR test results in the same time frame as the PD-L1 IHC test results, which is important as EGFR positivity may be a contra-indication for PD-1/PD-L1 therapy in non-small cell lung cancer (NSCLC)2.
The impact of the rapid Idylla™ EGFR Mutation Test on turnaround times is in line with previous studies3 published, concluding that Idylla™ testing early on may contribute to improving strategic treatment decisions in a multidisciplinary team for patients with NSCLC by the early screening of EGFR mutations.
References
1. Banyi N, Alex D, Hughesman C, McNeil K, N Ionescu D, Ma C, Yip S, Melosky B. Improving Time-to-Treatment for Advanced Non-Small Cell Lung Cancer Patients through Faster Single Gene EGFR Testing Using the Idylla™ EGFR Testing Platform. Curr Oncol. 2022 Oct 18;29(10):7900-7911. doi: 10.3390/curroncol29100624. PMID: 36290901
2. J. Mazieres et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Annals of Oncology 2019
3. Including Petiteau C, Robinet-Zimmermann G, Riot A, Dorbeau M, Richard N, Blanc-Fournier C, Bibeau F, Deshayes S, Bergot E, Gervais R, Levallet G. Contribution of the IdyllaTM System to Improving the Therapeutic Care of Patients with NSCLC through Early Screening of EGFR Mutations. Curr Oncol. 2021 Nov 3;28(6):4432-4445. doi: 10.3390/curroncol28060376. PMID: 34898548; PMCID: PMC8628756; Finall A, Davies G, Jones T, et al. J Clin Pathol Epub ahead of print. doi:10.1136/ jclinpath-2021-207987